Molecular Weight CalculatorThis online calculator you can use for computing the average molecular weight (MW) of molecules by entering the chemical formulas (for example C3H4OH(COOH)3 ). Or you can choose by one of the next two option-lists, which contains a series of common organic compounds (including their chemical formula) and all the elements. The molecular mass calculator will recognize the entered formula's, which are included in the list of organic compounds. !!!Lenntech BV cannot be held responsible for errors in the calculation,
J113 jfet. [ Calculators ] [ Converters ] [ Periodic Table ] |
More from 'Calculators'
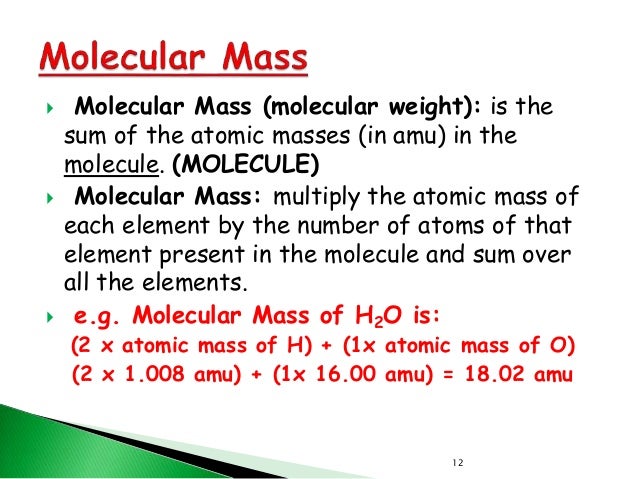
The proton mass is slightly less than the neutron mass. Mass of proton can be measured using the units kg, MeV/c, and u (AMU). Mass of the proton is the sum of the mass of current quarks and the binding gluons. The mass of the proton is-Proton mass m p = 1.672621898(21)×10 −27 Kg. Mass of proton is higher than the mass of electron. In C 12 H 10 O 6, there are 12 carbon atoms and atomic mass of one carbon atom is equal to 12.01 amu. Thus, the total mass (amu) of carbon in C 12 H 10 O 6 is calculated as follows: 12 × 12.01 amu = 144.12 amu. One Unified Atomic Mass Unit consists (AMU) Consists of 1.66054e − 24 grams of mass. AMU is the average of the proton rest mass and the neutron rest mass. Dmg mac os x download. This is approximately 1.67377 x 10 − 27 kilogram (kg), or 1.67377 x 10 − 24 gram (g).
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
Rosetta mac download snow leopard. 5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Mass Of 1 Amu Quizlet
Lenntech DMCC (Middle East)
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Mass Of 1 Amu Quizlet
Atomic Mass Of 1 Amu
Copyright © 1998-2021 Lenntech B.V. All rights reserved
